Hepatitis Delta Virus Stock Production and R&D Support Services
ImQuest has developed methodology for the production of high concentrated Hepatitis Delta Virus Stocks (HDV), to support in vitro and in vivo antiviral services. With expertise gained from years of experience in cell culture and the development of new antiviral agents, we propagate, concentrate, and characterize viruses at scales ranging from milliliters to liters, depending on client needs.
Our team has extensive experience in antiviral testing service for the development of novel therapeutic and prevention products for HDV, including antiviral testing services utilizing the Huh-END7 cell line for stable cell line analysis, as well as de novo infection models in hNTPC and primary human hepatocytes.
Savel valuable time ad resources, and contact us to learn more about our stock production to support your internal HDV research efforts. Our team is also availble to support your in vitro research and development needs.
Innovative Screening Platform: HDV Antiviral Testing
ImQuest has developed assays to expedite the screening of antiviral agents against multiple hepatitis D virus (HDV) replication. The assay can be customized to meet your development needs and determine compound mechanism of action. Assays for antiviral neutralization are available.
The platform has been optimized for standard cell lines (Huh-END7 and hNTCP) and can be tested in primary human hepatocytes (PHHs).
Applicable for traditional small molecule therapeutics and biologics, modified for neutralization based assays and antibody based therapeutics, and genomic editing-based therapeutic applications.
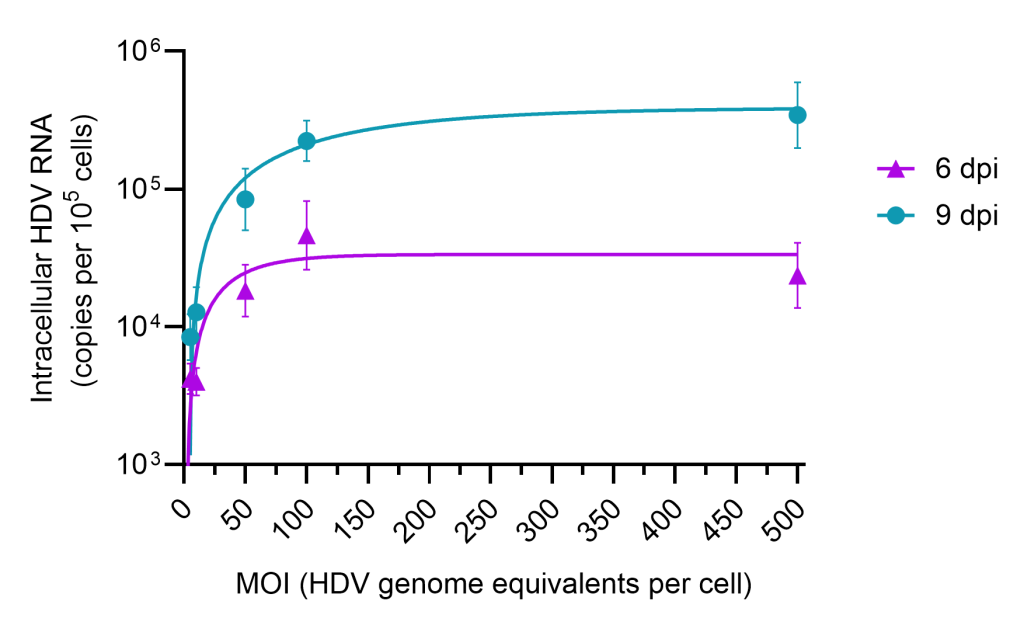
Currently, qPCR endpoints are available for monitoring intracellular HDV after test artcile treatment as well as immunofluorescent (IF) detection and quantification of infected hepatocytes.
Application include evaluation of viral neutralization, time of addition assessments, efficacy of drug combinations, selection and characterization of resistant strains and serum binding effects on antiviral efficacy.
Primary Human Hepatocyte Screening
Infected PHHs can be used to screen for antiviral compounds targeting HDV replication. The PHH system developed at ImQuest more closely resembles natural HDV infection and is the gold standard for antiviral agent screening.
High Titer HDV Virus Stock Production
ImQuest BioSciences provides technical services for the production of high titer and highly infectious HDV virus stocks. We can propagate, concentrate, and characterize HBV at scales ranging from milliliters to liters, saving you time and avoiding the tedious steps required for virus production. Whatever your needs, our scientists will customize the virus production process to your specifications.
Virus Production Services include:
- Production of crude virus preparations from Huh-END7 cell line.
- Concentration of virus from crude preparations utilizing Tangential Flow Filtration (TFF) if required
- Determination of virus concentration by qPCR and infectivity determination
- Preparation of inactivated virus
- Isolation of viral RNA
Documentation of final viral titer is provided with each lot of virus produced. Infectious virus in each pool may also be quantified by infectivity studies. Virus can be shipped domestically and internationally, and we are certified for the shipment of Category A and Category B Infectious Substances.
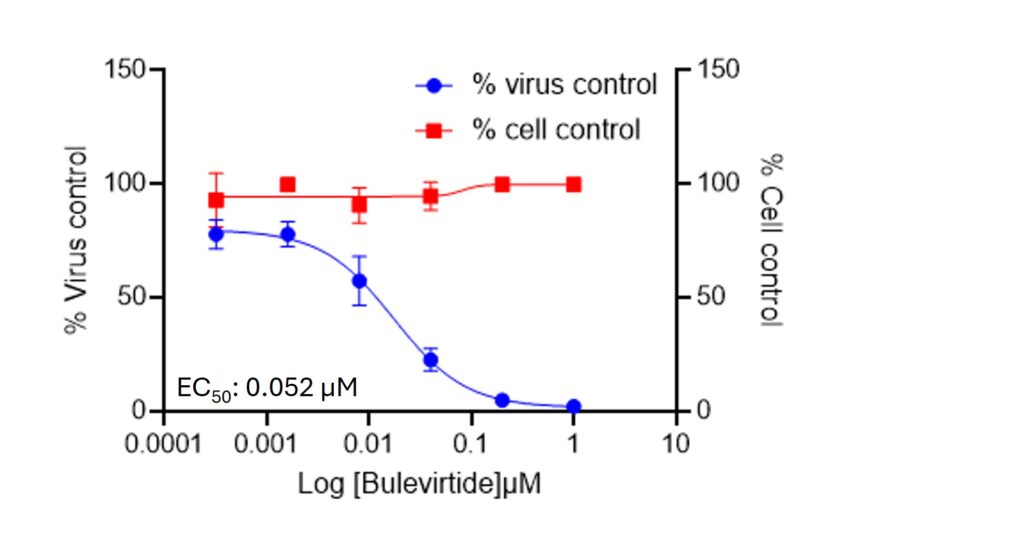
Hepatitis Delta Virus Infectivity and Antiviral Screening Platform
Request a Scientific Consultation
We would love to hear more about your research and development needs. Contact us and we can coordinate a discussion with one of our technical directors to better understand you needs and develop a customized solution.
Can’t find what you are looking for? Reach out to us, we can help.
Explore Additional Services
The ImQuest scientific team has extensive expertise in the development of small molecules, natural products, biologics and vaccines for the treatment and prevention of infectious disease, cancer, and inflammatory disease. Explore our additional service offering, or reach out to schedule a consultation.
Our Mission
Our goal is to help our clients accelerate their research and development efforts leading to the successful development of new products for the prevention and treatment of human disease. We have a very specific mission; accelerate your research and development plans and provide robust data in support of your projects.